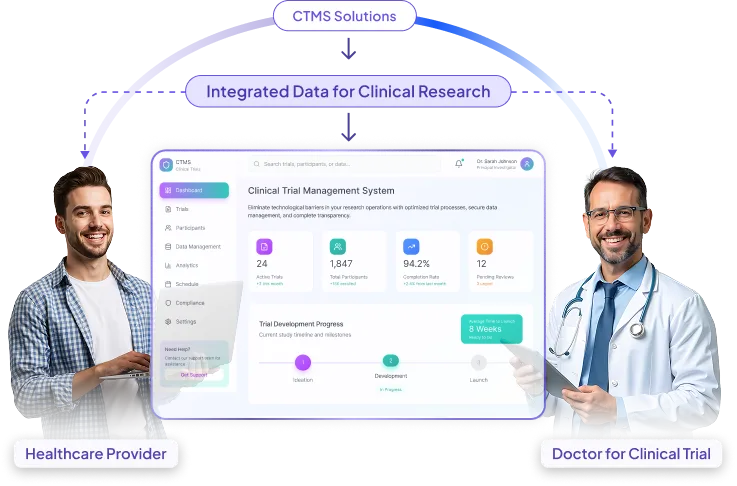















Improve visibility, speed up timelines, and strengthen collaboration with centralized trial intelligence powered by our clinical trial management software.

Manage multiple sponsor studies consistently with CTMS tools that boost efficiency and support seamless scaling across regions.

Less administrative work with easy visit tracking, document management, and payment tools, giving teams more time for patient care.
through automated workflows and site activation.
with integrated systems and a single source of truth.
with controlled access and audit-ready documentation.
by reducing manual tasks for CRAs, coordinators, and teams.
through automated budgeting & payments and reduced errors.
using real-time visibility into study status, risks, and bottlenecks.
with standardized processes across sites, vendors, and systems.
through configurable workflows that support complex, global studies.

Backed by 19+ years of experience in digital health, we bring deep domain knowledge to the clinical trial management solutions we develop.

We offer accelerated timelines with AI-driven workflow structures for data capture, monitoring, financial management, and clinical trial management in one intelligent platform.

Our clinical trial optimization software is designed through our healthcare app development expertise and is fully customized to your protocols and SOPs.

API-first architecture and native support for HL7/FHIR interface engines ensure smooth, secure data flow across all your systems.


Team Folio3 brought my dream app to reality, they explained every step to our non technical team professionally. wireframe and design processes were outstanding & they built stunning and perfectly integrated application. I think both patients and clinic will be significantly benefited.





Working with the folio3 team has been a great experience. Your dedication, creativity, and adaptability in overcoming challenges for the Moodology app project are truly commendable.


I appreciate your remarkable work during the recent Laboratory service phase. Your determination, analytical thinking, and continuous efforts were crucial in overcoming challenges and achieving success. I appreciate your help as we collaborate to create fantastic items.

Kudos to your team for outstanding work in the discovery phase! Your team understood our business and workflows by asking the right questions and extracting the most useful information. Your team's ownership and results-oriented approach are commendable.