






Our medical device software development company rigorously validates medical software under the strict compliance measures set by the FDA. Make sure your medical device software meets the quality standards by running automated performance and cybersecurity tests with our help.
Our AI-powered diagnostics tool reduced diagnosis time. It uses machine learning algorithms for early detection of skin diseases, improving both accuracy and speed in clinical workflows.
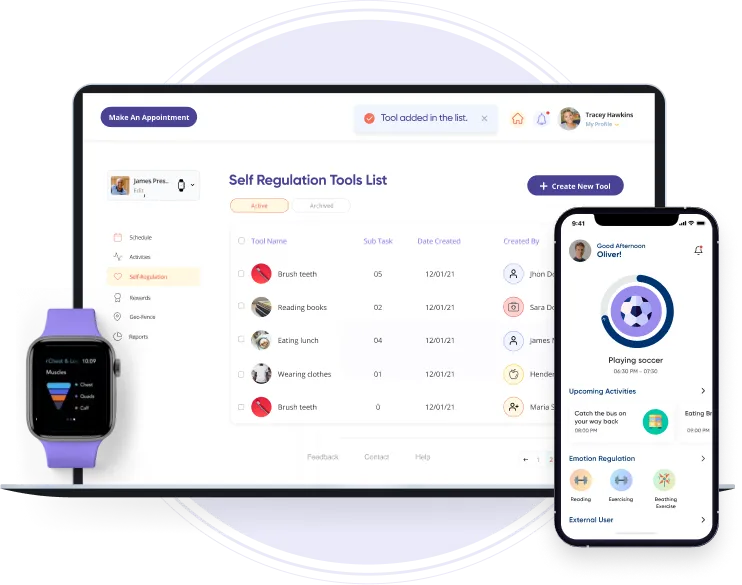
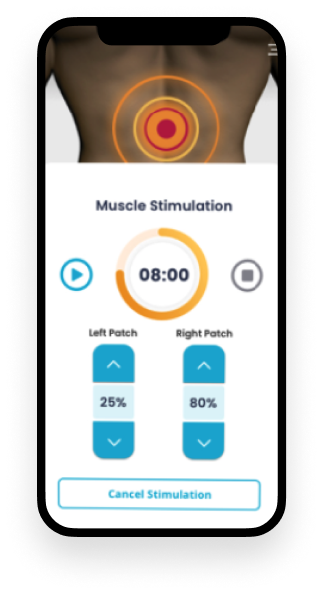
A companion application for two wearable devices: the Pain Relief Controller and the Smart Patch. Its intuitive interface enabled users to read sensor data from the devices easily. The integration allowed users to monitor pain relief progress and adjust settings accordingly.
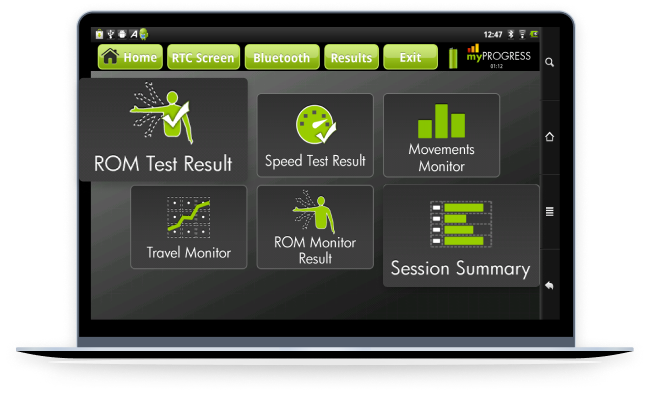
An Android companion app for a portable neuro-robotic arm brace designed to help patients with partially paralyzed limbs regain control over movement. The app allows users to initiate and control movements using their biological signals, facilitating real-time rehabilitation.
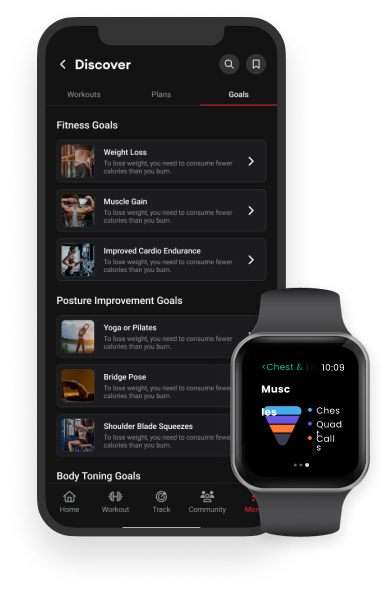
A companion platform for fitness equipment, such as the "Vahana 360" exercise machines, It allows users to track their workouts, schedule exercise sessions, and visualize their fitness goals. The personalized recommendations and predictive analytics monitored fatigue and optimized workout efficiency.








We study the market, evaluate viability, and create early prototypes. To ensure a smooth transition from conception to development, a strong regulatory strategy is created to satisfy all compliance requirements.
Our group develops and designs medical software by IEC 62304 criteria. To make sure the product is safe, useful, and compliant, we draft thorough project roadmaps and solicit input.
Throughout the software lifecycle, we verify and validate designs to make sure all performance, security, and compliance parameters are satisfied.
Once development is complete, we handle product deployment and provide continuous maintenance, including frequent updates, bug fixes, and post-launch support to ensure long-term compliance and performance.